Sticking it to the man
#141
I suspect part of the problem is that, since you are indeed using hot water, it has already reached an elevated mineral level in the process heating. You can usually taste the difference between your hot and cold tap water, which is the main reason why you never start with hot water for making pasta, soup, coffee, etc..
Perhaps, as a cleaning regimen, you could just periodically run the radiator in a close loop with an (aquarium pump or something) while filled with a vinegar or CLR solution.
Perhaps, as a cleaning regimen, you could just periodically run the radiator in a close loop with an (aquarium pump or something) while filled with a vinegar or CLR solution.
#142
Elite Member

iTrader: (3)
Join Date: Apr 2008
Location: Outside Portland Maine
Posts: 2,023
Total Cats: 19
The hot water would certainly carry a lot more dissolved minerals, and would deposit them as it cools. I suspect your best solution would be to clean it periodically somehow. I am no expert in that though.
#143
Calcium is like impossible to get off. You need a freakin chisel.
You are in an apartment? Do you have a balcony? Can you do something like this? How to Build a DIY Solar Air Heater from Old Soda Cans : TreeHugger
You are in an apartment? Do you have a balcony? Can you do something like this? How to Build a DIY Solar Air Heater from Old Soda Cans : TreeHugger
#145
Boost Pope


Thread Starter
iTrader: (8)
Join Date: Sep 2005
Location: Chicago. (The less-murder part.)
Posts: 33,027
Total Cats: 6,593
You pass the beer through the copper tubing from top-to-bottom, and pump sink-temperature water through the hose from bottom-to-top. Once you get it set up correctly, the beer exits the bottom at nearly room-temperature.
Looks like this:

Sadly, I parted company with it when I left CA.
However, I have had an idea.
This past weekend, as with most of late, I was at the woman's place up in the Catskills. She retains a modest little 1br cottage out in the woods, with deer prancing by the front window and whatnot. Very rustic.

(It's surprisingly inexpensive, and I highly recommend it to anyone who has the opportunity. As a sidebar, I have come to learn that I am what the locals refer to as a Citiot, being a portmanteau of City + Idiot. Eg: a resident of New York City who spends his weekends contaminating the small towns of upstate New York like something out of My Cousin Vinny. Well, guilty as charged.)
A minor part of said rusticism is a complete absence of common municipal utilities such as water and sewer. (This is a rather interesting and unique concept for us city-folk.)
So this past Saturday, as I was performing field expedient repairs upon the pressure-equalizing tank on the well pump (a ruptured diaphragm caused the system to gradually go solid, with attendant short-cycling of the pump and irregular water pressure), I happened to notice for the first time that the house has no furnace.
I mean, I was cognizant of this previously, but the implications of it had failed entirely to register with me. The house is heated entirely by baseboard radiators, supplied by the same boiler which provides for the potable domestic hot water system. What I mean is that I really grokked this in fullness.
In other words, this house is equipped with the exact thing that I have been attempting to accomplish!
Apparently, this is actually a common thing up here in the frigid northeast. It's a concept with which I was completely unfamiliar until just recently, but I gather that the idea of passing hot water through a radiator as a means of heating the home is not nearly so novel as I had originally thought, to the point where several different companies actually produce radiators intended specifically for this purpose, and with copper cores, no less.
Examples:
Shop Hydrotherm 8-ft Hydronic Baseboard Heater Enclosure at Lowes.com

Slant/Fin Fine/Line 30 6 ft. Hydronic Baseboard Fully Assembled Enclosure and Element-101-401-6 at The Home Depot

I believe that I may give one of these a try. An 8' unit would fit nicely in the bedroom to the side of the bed, and if it turns out to be a viable option, I could easily accommodate a second 8' strip in the living room behind the sofa. Collectively, those would give me a lot more surface area than I had with the little go-kart radiator, which should make up for the lack of forced-air circulation.
If I really decide to get fancy, I can source bedroom water from the bathroom, and living-room water from the kitchen, and thus avoid having a hose strung across the hallway altogether.
Now, all I need to do is figure out how to transport a pair of 8' long radiators on a bicycle.
Last edited by Joe Perez; 02-11-2014 at 12:07 AM.
#146
Ah hell Joe, here's the damn wiki:
Limescale is the hard, off-white, chalky deposit found in kettles, hot-water boilers and the inside of inadequately maintained hot-water central heating systems. It is also often found as a similar deposit on the inner surface of old pipes and other surfaces where "hard water" has evaporated. Other than being unsightly and harder to clean, limescale seriously impairs the operation or damages various components.[1]
The type found deposited on the heating elements of water heaters has a main component of calcium carbonate. Hard water contains calcium (and often magnesium) bicarbonate and/or similar ions. Calcium salts, such as calcium bicarbonate and calcium carbonate are both more soluble in hot water than cold water. Thus, heating water does not cause calcium carbonate to precipitate per se. However, there is an equilibrium between dissolved calcium bicarbonate and dissolved calcium carbonate:
Ca2+ + 2HCO3- ⇋ Ca2+ + CO32- + CO2 + H2O
where the equilibrium is driven by the carbonate/bicarbonate, not the calcium. Note that the CO2 is dissolved in the water.
There is also an equilibrium of carbon dioxide between dissolved in water (dis) and the gaseous state (g): CO2(dis) ⇋ CO2(g)
The equilibrium of CO2 also moves to the right towards gaseous CO2 when the water temperature rises. When water that contains dissolved calcium carbonate is warmed, CO2 is removed from the water as gas causing the equilibrium of bicarbonate and carbonate to shift to the right, increasing the concentration of dissolved carbonate. As the concentration of carbonate increases, calcium carbonate precipitates as the salt: Ca2+ + CO32- ⇋ CaCO3.
As new cold water with dissolved calcium carbonate/bicarbonate is added and heated, CO2 gas is removed, carbonate concentration increases, and more calcium carbonate precipitates.
#147
Boost Pope


Thread Starter
iTrader: (8)
Join Date: Sep 2005
Location: Chicago. (The less-murder part.)
Posts: 33,027
Total Cats: 6,593
There is also an equilibrium of carbon dioxide between dissolved in water (dis) and the gaseous state (g): CO2(dis) ⇋ CO2(g)
The equilibrium of CO2 also moves to the right towards gaseous CO2 when the water temperature rises. When water that contains dissolved calcium carbonate is warmed, CO2 is removed from the water as gas causing the equilibrium of bicarbonate and carbonate to shift to the right, increasing the concentration of dissolved carbonate. As the concentration of carbonate increases, calcium carbonate precipitates as the salt: Ca2+ + CO32- ⇋ CaCO3.
As new cold water with dissolved calcium carbonate/bicarbonate is added and heated, CO2 gas is removed, carbonate concentration increases, and more calcium carbonate precipitates.
The equilibrium of CO2 also moves to the right towards gaseous CO2 when the water temperature rises. When water that contains dissolved calcium carbonate is warmed, CO2 is removed from the water as gas causing the equilibrium of bicarbonate and carbonate to shift to the right, increasing the concentration of dissolved carbonate. As the concentration of carbonate increases, calcium carbonate precipitates as the salt: Ca2+ + CO32- ⇋ CaCO3.
As new cold water with dissolved calcium carbonate/bicarbonate is added and heated, CO2 gas is removed, carbonate concentration increases, and more calcium carbonate precipitates.
See, I knew I needed to consult a chemist here.
I'm curious on a twofold level here...
A: If I'm interpreting that correctly, then the precipitation of solid calcium carbonate should occur within the boiler during the heating process, not in my radiator during the cooling process, especially since the system is essentially closed between the boiler and the radiator, with no obvious source for gaseous CO2 to be re-absorbed.
B: Why does the aluminum radiator seem to be uniquely sensitive to this precipitation, while the copper plumbing of the building has survived several decades without becoming completely plugged up?
#148
B: Why does the aluminum radiator seem to be uniquely sensitive to this precipitation, while the copper plumbing of the building has survived several decades without becoming completely plugged up?
#149
Elite Member

iTrader: (5)
Join Date: Oct 2011
Location: Detroit (the part with no rules or laws)
Posts: 5,677
Total Cats: 800
I just had the chemist guy here yesterday testing the water in our closed loop hot water system here. I now wish i could ask him questions.
I'm not a chemist, or even a boiler guy. But i am in a boiler room now. What i do know is that every heat exchanger happens to have copper pipes (with aluminum fins). All the hot water passes through regular galvanized pipe until it reaches the heat exhanger when it is changed to copper. Sometimes brass fittings are used too. I also know the PH level for the system is checked every month(by the guy that was here yesterday). I'm positive we have a softner that fixes the domestic water before it enters the closed loop system.
To answer B. "In areas with neutral-pH water, copper pipes resist corrosion by forming a thin protective coating inside of the pipe and keep their structural integrity well."
Aluminum doesn't do that, no idea why.
I'm not a chemist, or even a boiler guy. But i am in a boiler room now. What i do know is that every heat exchanger happens to have copper pipes (with aluminum fins). All the hot water passes through regular galvanized pipe until it reaches the heat exhanger when it is changed to copper. Sometimes brass fittings are used too. I also know the PH level for the system is checked every month(by the guy that was here yesterday). I'm positive we have a softner that fixes the domestic water before it enters the closed loop system.
To answer B. "In areas with neutral-pH water, copper pipes resist corrosion by forming a thin protective coating inside of the pipe and keep their structural integrity well."
Aluminum doesn't do that, no idea why.
#150
2 Props,3 Dildos,& 1 Cat


iTrader: (8)
Join Date: Jun 2005
Location: Fake Virginia
Posts: 19,338
Total Cats: 573
I would still go to home depot and get the carbon whole-house filter and try it. It is not a softener, but DID do a good job of removing crap in my water that was precipitating out in the form of white gunk and pink gunk.
#151
Boost Pope


Thread Starter
iTrader: (8)
Join Date: Sep 2005
Location: Chicago. (The less-murder part.)
Posts: 33,027
Total Cats: 6,593
I have decided that it is time for me to stop tip-toeing around the issue, and that I am best served to simply take off and nuke this problem from orbit.
Parts have been ordered, more details should be forthcoming within a day or two.
Parts have been ordered, more details should be forthcoming within a day or two.
#152
Moderator


iTrader: (12)
Join Date: Nov 2008
Location: Tampa, Florida
Posts: 20,652
Total Cats: 3,011
FWIW, copper pipes in homes in certain areas around here are dissolved by the hard tap water. They become paper thin and fail within 20 years unless you have a softener. That can be exciting when the slab or drywall erupts. They then replumb the house with pvc through the attic.
That reminds me, I'm due to replace the sacrificial anode in my water heater.
That reminds me, I'm due to replace the sacrificial anode in my water heater.
#153
Boost Pope


Thread Starter
iTrader: (8)
Join Date: Sep 2005
Location: Chicago. (The less-murder part.)
Posts: 33,027
Total Cats: 6,593
FWIW, copper pipes in homes in certain areas around here are dissolved by the hard tap water. They become paper thin and fail within 20 years unless you have a softener. That can be exciting when the slab or drywall erupts. They then replumb the house with pvc through the attic.
My mother's new house, which was built around 2000 or so, has rigid CPVC in the attic. And from what I gather, they're actually staring to use PEX in a lot of new-construction, which should be even better (resistant to bursting when frozen, extremely easy to de-mate and re-terminate, etc.)
I lived in a house with a water-softener in 2003-4, and I have no desire to ever repeat that experience. I just detest that slick, slimy feeling that you get in the shower, wherein you can never quite tell if you're actually clean and rinsed off or not.
#154
Senior Member
iTrader: (1)
Join Date: Dec 2008
Location: Manassas, Virginia
Posts: 1,242
Total Cats: 57
Apparently, this is actually a common thing up here in the frigid northeast. It's a concept with which I was completely unfamiliar until just recently, but I gather that the idea of passing hot water through a radiator as a means of heating the home is not nearly so novel as I had originally thought, to the point where several different companies actually produce radiators intended specifically for this purpose, and with copper cores, no less.
#155
Boost Pope


Thread Starter
iTrader: (8)
Join Date: Sep 2005
Location: Chicago. (The less-murder part.)
Posts: 33,027
Total Cats: 6,593
When you've spent most of your life in Florida, Southern California and the Caribbean, you aren't exposed to a lot of different concepts in home heating. I did live in Ohio for several years, but my house there had a ducted, forced-air heating system which was functionally identical to what I'd experienced elsewhere, only with nat-gas rather than electricity as the primary source of thermal energy.
While I am vaguely familiar with the notion of the old cast-iron radiators through which steam is allowed to pass, it had never occurred to me that pumping hot, potable water through finned radiators was actually a common thing in "normal" homes of the present day in north America.
#157
Boost Pope


Thread Starter
iTrader: (8)
Join Date: Sep 2005
Location: Chicago. (The less-murder part.)
Posts: 33,027
Total Cats: 6,593
Does Catskill / Hudson / Cairo not count as getting out?!
I'm not sure how much more "out" it's possible to get than this, short of exiting the Earth's atmosphere.

If you just mean exposure to different heating systems, then I'm sorry if I'm not as well-versed in 19th century HVAC technology as some people. Down south, we worry more about not dying of heat-stroke in the summer than about how to keep warm on those frigid 50° nights.
I'm not sure how much more "out" it's possible to get than this, short of exiting the Earth's atmosphere.

If you just mean exposure to different heating systems, then I'm sorry if I'm not as well-versed in 19th century HVAC technology as some people. Down south, we worry more about not dying of heat-stroke in the summer than about how to keep warm on those frigid 50° nights.

#159
Elite Member

iTrader: (5)
Join Date: Oct 2011
Location: Detroit (the part with no rules or laws)
Posts: 5,677
Total Cats: 800
This is sorta on topic.
You would be shocked to find what's in your potable water.
The building i work in is pretty dang old, i'm not sure how old, but i do know it's been through more than one renovation. During the last one the newest hot water heater was installed. More than likely by a 3 year old.
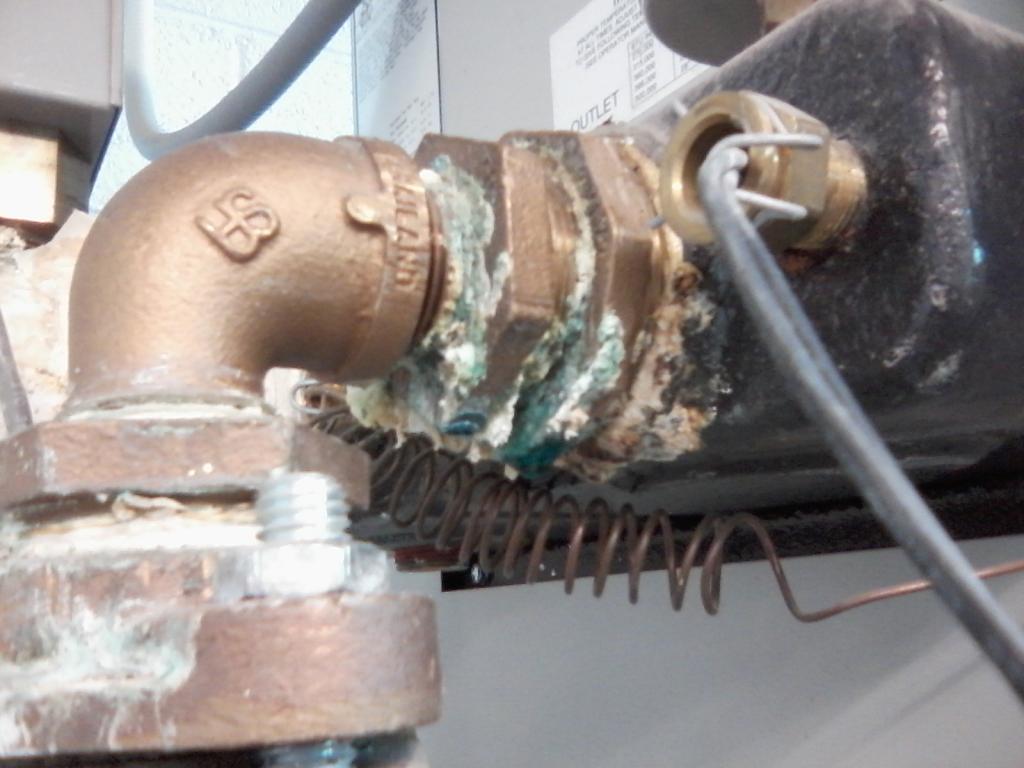

We keep our hot water at 150F which is way to damn hot, but OSHA or something.
It has been reeking havoc on the pipes, which is on the plate for a job i need to do here soon. Though it's hard to shut an entire buildings hot water off for an extended period of time.
Now i had to laugh a little at the post about deteriorating copper pipes.
This is what happens when you pass 140F water though galvanized pipes.

Now take a guess at how often i have to replace galvanized pipe that's sprung a leak.

Most recently the main coming into the building.
Which could throw me into a "Joe Perez" style rant about how the infrastructure of this country is getting worse and worse by the day and nothing is being done about it.
You would be shocked to find what's in your potable water.
The building i work in is pretty dang old, i'm not sure how old, but i do know it's been through more than one renovation. During the last one the newest hot water heater was installed. More than likely by a 3 year old.


We keep our hot water at 150F which is way to damn hot, but OSHA or something.
It has been reeking havoc on the pipes, which is on the plate for a job i need to do here soon. Though it's hard to shut an entire buildings hot water off for an extended period of time.
Now i had to laugh a little at the post about deteriorating copper pipes.
This is what happens when you pass 140F water though galvanized pipes.

Now take a guess at how often i have to replace galvanized pipe that's sprung a leak.

Most recently the main coming into the building.
Which could throw me into a "Joe Perez" style rant about how the infrastructure of this country is getting worse and worse by the day and nothing is being done about it.