The AI-generated cat pictures thread
Boost Pope


iTrader: (8)
Join Date: Sep 2005
Location: Chicago. (The less-murder part.)
Posts: 33,027
Total Cats: 6,593
Pressure Cooker
Pressure cookers are dangerous.
They can explode, in a sense, but not as violently as you might fear (or hope). The pressure inside a consumer cooker doesn’t go above about two atmospheres—about the pressure inside a can of soda. Those levels can be dangerous, but they’re generally not high enough to cause the metal to violently rupture.
So what makes a pressure cooker dangerous?
Imagine a world where Pepsi is scalding hot. Now imagine that someone shakes up a can of Pepsi and sets it in front of you.
That’s the real threat from a pressure cooker: If the seal fails (or the lid is opened too early), it can spray scalding stew in all directions.
But it’s not really an explosion.
The blast couldn’t even fling the lid very far. If you mounted a rifle-style barrel on a pressure cooker, even in ideal circumstances it wouldn’t be able to fire the lid much faster than you could throw it. Any potato cannon (especially this one) could do better.
Of course, the question wasn’t about whether a pressure cooker is likely to explode. It was about what the worst thing that could happen was.
If you disable the safety valve, there are plenty of ways to produce much more dangerous pressures. You could completely fill it with water and heat it, fill it with Drano and aluminum foil, or just pump in air from a compressor.
The result would depend on your pressure cooker. Chances are it would start to leak. If it didn’t, and it somehow stayed together up a few hundred atmospheres (pressures typical of scuba tank), when it finally ruptured it could easily kill you.
Even so, that’s far from the worst thing you could do with a pressure cooker.
Frankly, there are so many options it would be impossible to survey them all. But for my money, one of the most horrifying things you could do is this:
(Note: Never try this, for reasons which will become obvious in a moment.)
Fill the cooker with oxygen up to 5 PSI, then pump in fluorine until it starts escaping through the safety valve. Put the vessel over an open flame until it reaches 700°C (That’s °C, not °F. Yes, this will probably set off the smoke alarm.) Now, pump the hot gas over a liquid-oxygen-cooled stainless steel surface.
The procedure here is a little tricky, but if you do things right, the gas will condense into dioxygen difluoride (O2F2).
And that stuff is awful.
Ray Bradbury taught us that paper burns when exposed to oxygen at temperatures above 451°F. Dioxygen difluoride is so volatile that it makes almost any organic substance ignite and explode at any temperature hotter than 300°F below zero. It can literally make ice catch fire.
In an article about O2F2, Chemistry blogger Derek Lowe (of the excellent In The Pipeline) used phrases like “violently hideous”, “deeply alarming”, and “chemicals that I never hope to encounter”. Another article refers to fluorine as “the gas of Lucifer”, and lists chemists who were poisoned or blown up while attempting to work with it.
If your house is heated by natural gas, and it happens to contain hydrogen sulfide, you could pipe some of it into your container of O2F2. In addition to a massive explosion, this will also produce a cloud of hydrogen fluoride gas. Hydrogen fluoride can dissolve human tissue on contact, starting with your lungs and corneas.
As Lowe points out, the chemistry of this kind of reaction (O2F2 and sulfides) is largely unexplored.
Which gives us an answer to our question. What’s the worst thing that can happen in a pressure cooker?
Science!
Am I right to be afraid of pressure cookers? What's the worst thing that can happen if you misuse a pressure cooker in an ordinary kitchen?
—Delphine Lourtau
The worst thing?—Delphine Lourtau

Pressure cookers are dangerous.
They can explode, in a sense, but not as violently as you might fear (or hope). The pressure inside a consumer cooker doesn’t go above about two atmospheres—about the pressure inside a can of soda. Those levels can be dangerous, but they’re generally not high enough to cause the metal to violently rupture.
So what makes a pressure cooker dangerous?
Imagine a world where Pepsi is scalding hot. Now imagine that someone shakes up a can of Pepsi and sets it in front of you.

That’s the real threat from a pressure cooker: If the seal fails (or the lid is opened too early), it can spray scalding stew in all directions.
But it’s not really an explosion.
The blast couldn’t even fling the lid very far. If you mounted a rifle-style barrel on a pressure cooker, even in ideal circumstances it wouldn’t be able to fire the lid much faster than you could throw it. Any potato cannon (especially this one) could do better.
Of course, the question wasn’t about whether a pressure cooker is likely to explode. It was about what the worst thing that could happen was.
If you disable the safety valve, there are plenty of ways to produce much more dangerous pressures. You could completely fill it with water and heat it, fill it with Drano and aluminum foil, or just pump in air from a compressor.
The result would depend on your pressure cooker. Chances are it would start to leak. If it didn’t, and it somehow stayed together up a few hundred atmospheres (pressures typical of scuba tank), when it finally ruptured it could easily kill you.

Even so, that’s far from the worst thing you could do with a pressure cooker.
Frankly, there are so many options it would be impossible to survey them all. But for my money, one of the most horrifying things you could do is this:
(Note: Never try this, for reasons which will become obvious in a moment.)
Fill the cooker with oxygen up to 5 PSI, then pump in fluorine until it starts escaping through the safety valve. Put the vessel over an open flame until it reaches 700°C (That’s °C, not °F. Yes, this will probably set off the smoke alarm.) Now, pump the hot gas over a liquid-oxygen-cooled stainless steel surface.
The procedure here is a little tricky, but if you do things right, the gas will condense into dioxygen difluoride (O2F2).
And that stuff is awful.
Ray Bradbury taught us that paper burns when exposed to oxygen at temperatures above 451°F. Dioxygen difluoride is so volatile that it makes almost any organic substance ignite and explode at any temperature hotter than 300°F below zero. It can literally make ice catch fire.
In an article about O2F2, Chemistry blogger Derek Lowe (of the excellent In The Pipeline) used phrases like “violently hideous”, “deeply alarming”, and “chemicals that I never hope to encounter”. Another article refers to fluorine as “the gas of Lucifer”, and lists chemists who were poisoned or blown up while attempting to work with it.
If your house is heated by natural gas, and it happens to contain hydrogen sulfide, you could pipe some of it into your container of O2F2. In addition to a massive explosion, this will also produce a cloud of hydrogen fluoride gas. Hydrogen fluoride can dissolve human tissue on contact, starting with your lungs and corneas.
As Lowe points out, the chemistry of this kind of reaction (O2F2 and sulfides) is largely unexplored.
Which gives us an answer to our question. What’s the worst thing that can happen in a pressure cooker?

Science!
Tour de Franzia

iTrader: (6)
Join Date: Jun 2006
Location: Republic of Dallas
Posts: 29,085
Total Cats: 375
This link down here is art but its most likely NWS so click at your own risk.
https://i.imgur.com/LxD9Wvu.jpg
I would buy that if I could.
https://i.imgur.com/LxD9Wvu.jpg
I would buy that if I could.
Pressure Cooker

Pressure cookers are dangerous.
They can explode, in a sense, but not as violently as you might fear (or hope). The pressure inside a consumer cooker doesn’t go above about two atmospheres—about the pressure inside a can of soda. Those levels can be dangerous, but they’re generally not high enough to cause the metal to violently rupture.
So what makes a pressure cooker dangerous?
Imagine a world where Pepsi is scalding hot. Now imagine that someone shakes up a can of Pepsi and sets it in front of you.

That’s the real threat from a pressure cooker: If the seal fails (or the lid is opened too early), it can spray scalding stew in all directions.
But it’s not really an explosion.
The blast couldn’t even fling the lid very far. If you mounted a rifle-style barrel on a pressure cooker, even in ideal circumstances it wouldn’t be able to fire the lid much faster than you could throw it. Any potato cannon (especially this one) could do better.
Of course, the question wasn’t about whether a pressure cooker is likely to explode. It was about what the worst thing that could happen was.
If you disable the safety valve, there are plenty of ways to produce much more dangerous pressures. You could completely fill it with water and heat it, fill it with Drano and aluminum foil, or just pump in air from a compressor.
The result would depend on your pressure cooker. Chances are it would start to leak. If it didn’t, and it somehow stayed together up a few hundred atmospheres (pressures typical of scuba tank), when it finally ruptured it could easily kill you.

Even so, that’s far from the worst thing you could do with a pressure cooker.
Frankly, there are so many options it would be impossible to survey them all. But for my money, one of the most horrifying things you could do is this:
(Note: Never try this, for reasons which will become obvious in a moment.)
Fill the cooker with oxygen up to 5 PSI, then pump in fluorine until it starts escaping through the safety valve. Put the vessel over an open flame until it reaches 700°C (That’s °C, not °F. Yes, this will probably set off the smoke alarm.) Now, pump the hot gas over a liquid-oxygen-cooled stainless steel surface.
The procedure here is a little tricky, but if you do things right, the gas will condense into dioxygen difluoride (O2F2).
And that stuff is awful.
Ray Bradbury taught us that paper burns when exposed to oxygen at temperatures above 451°F. Dioxygen difluoride is so volatile that it makes almost any organic substance ignite and explode at any temperature hotter than 300°F below zero. It can literally make ice catch fire.
In an article about O2F2, Chemistry blogger Derek Lowe (of the excellent In The Pipeline) used phrases like “violently hideous”, “deeply alarming”, and “chemicals that I never hope to encounter”. Another article refers to fluorine as “the gas of Lucifer”, and lists chemists who were poisoned or blown up while attempting to work with it.
If your house is heated by natural gas, and it happens to contain hydrogen sulfide, you could pipe some of it into your container of O2F2. In addition to a massive explosion, this will also produce a cloud of hydrogen fluoride gas. Hydrogen fluoride can dissolve human tissue on contact, starting with your lungs and corneas.
As Lowe points out, the chemistry of this kind of reaction (O2F2 and sulfides) is largely unexplored.
Which gives us an answer to our question. What’s the worst thing that can happen in a pressure cooker?

Science!
Am I right to be afraid of pressure cookers? What's the worst thing that can happen if you misuse a pressure cooker in an ordinary kitchen?
—Delphine Lourtau
The worst thing?—Delphine Lourtau

Pressure cookers are dangerous.
They can explode, in a sense, but not as violently as you might fear (or hope). The pressure inside a consumer cooker doesn’t go above about two atmospheres—about the pressure inside a can of soda. Those levels can be dangerous, but they’re generally not high enough to cause the metal to violently rupture.
So what makes a pressure cooker dangerous?
Imagine a world where Pepsi is scalding hot. Now imagine that someone shakes up a can of Pepsi and sets it in front of you.

That’s the real threat from a pressure cooker: If the seal fails (or the lid is opened too early), it can spray scalding stew in all directions.
But it’s not really an explosion.
The blast couldn’t even fling the lid very far. If you mounted a rifle-style barrel on a pressure cooker, even in ideal circumstances it wouldn’t be able to fire the lid much faster than you could throw it. Any potato cannon (especially this one) could do better.
Of course, the question wasn’t about whether a pressure cooker is likely to explode. It was about what the worst thing that could happen was.
If you disable the safety valve, there are plenty of ways to produce much more dangerous pressures. You could completely fill it with water and heat it, fill it with Drano and aluminum foil, or just pump in air from a compressor.
The result would depend on your pressure cooker. Chances are it would start to leak. If it didn’t, and it somehow stayed together up a few hundred atmospheres (pressures typical of scuba tank), when it finally ruptured it could easily kill you.

Even so, that’s far from the worst thing you could do with a pressure cooker.
Frankly, there are so many options it would be impossible to survey them all. But for my money, one of the most horrifying things you could do is this:
(Note: Never try this, for reasons which will become obvious in a moment.)
Fill the cooker with oxygen up to 5 PSI, then pump in fluorine until it starts escaping through the safety valve. Put the vessel over an open flame until it reaches 700°C (That’s °C, not °F. Yes, this will probably set off the smoke alarm.) Now, pump the hot gas over a liquid-oxygen-cooled stainless steel surface.
The procedure here is a little tricky, but if you do things right, the gas will condense into dioxygen difluoride (O2F2).
And that stuff is awful.
Ray Bradbury taught us that paper burns when exposed to oxygen at temperatures above 451°F. Dioxygen difluoride is so volatile that it makes almost any organic substance ignite and explode at any temperature hotter than 300°F below zero. It can literally make ice catch fire.
In an article about O2F2, Chemistry blogger Derek Lowe (of the excellent In The Pipeline) used phrases like “violently hideous”, “deeply alarming”, and “chemicals that I never hope to encounter”. Another article refers to fluorine as “the gas of Lucifer”, and lists chemists who were poisoned or blown up while attempting to work with it.
If your house is heated by natural gas, and it happens to contain hydrogen sulfide, you could pipe some of it into your container of O2F2. In addition to a massive explosion, this will also produce a cloud of hydrogen fluoride gas. Hydrogen fluoride can dissolve human tissue on contact, starting with your lungs and corneas.
As Lowe points out, the chemistry of this kind of reaction (O2F2 and sulfides) is largely unexplored.
Which gives us an answer to our question. What’s the worst thing that can happen in a pressure cooker?

Science!

Fill the cooker with oxygen up to 5 PSI, then pump in fluorine until it starts escaping through the safety valve. Put the vessel over an open flame until it reaches 700°C (That’s °C, not °F. Yes, this will probably set off the smoke alarm.) Now, pump the hot gas over a liquid-oxygen-cooled stainless steel surface.
The procedure here is a little tricky, but if you do things right, the gas will condense into dioxygen difluoride (O2F2).
And that stuff is awful.
The procedure here is a little tricky, but if you do things right, the gas will condense into dioxygen difluoride (O2F2).
And that stuff is awful.

Boost Pope


iTrader: (8)
Join Date: Sep 2005
Location: Chicago. (The less-murder part.)
Posts: 33,027
Total Cats: 6,593
I'd never really done much reading about fluorides, but it turns out that they're a really fascinating branch of chemistry.
I'll give you an example: Chlorine trifluoride.
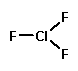
Now, Wikipedia is not generally known for hyperbole (or even having a sense of humor) but I find its description of chlorine trifluoride to be utterly hilarious. Here's an excerpt:
I'll give you an example: Chlorine trifluoride.
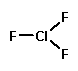
Now, Wikipedia is not generally known for hyperbole (or even having a sense of humor) but I find its description of chlorine trifluoride to be utterly hilarious. Here's an excerpt:
ClF3 is a very strong oxidizing and fluorinating agent. It is extremely reactive with most inorganic and organic materials — this includes glass and teflon —, and will initiate the combustion of many otherwise non-flammable materials without any ignition source. These reactions are often violent, and in some cases explosive. Vessels made from steel, copper or nickel resist the attack of the material due to formation of a thin layer of insoluble metal fluoride, but molybdenum, tungsten and titanium form volatile fluorides and are consequently unsuitable. Any equipment that comes into contact with chlorine trifluoride must be scrupulously cleaned and then passivated, because any contamination left may burn through the passivation layer faster than it can re-form.
The ability to surpass the oxidizing ability of oxygen leads to extreme corrosivity against oxide-containing materials often thought as incombustible. Chlorine trifluoride and gases like it have been reported to ignite sand, asbestos, and other highly fire-retardant materials. In an industrial accident, a spill of 900 kg of chlorine trifluoride burned through 30 cm of concrete and 90 cm of gravel beneath.[12] Fire control/suppression is incapable of suppressing this oxidation, therefore the surrounding area is kept cool until the reaction ceases.[13] The compound reacts violently with water-based suppressors, and oxidizes in the absence of atmospheric oxygen, rendering atmosphere-displacement suppressors such as CO2 and halon completely ineffective. It ignites glass on contact.[14]
Exposure of larger amounts of chlorine trifluoride, as a liquid or as a gas, ignites tissue. The hydrolysis reaction with water is violent and exposure results in a thermal burn. The products of hydrolysis are mainly hydrofluoric acid and hydrochloric acid, usually released as steam or vapor due to the highly exothermic nature of the reaction. Hydrofluoric acid is corrosive to human tissue, is absorbed through skin, selectively attacks bone and stimulates pain nerves, and causes often-fatal fluorine poisoning. Hydrochloric acid is secondary in its danger to living organisms, but is several times more corrosive to most inorganic materials than hydrofluoric acid.
Chlorine trifluoride has been investigated as a high-performance storable oxidizer in rocket propellant systems. Handling concerns, however, prevented its use. John D. Clark summarized the difficulties:
The ability to surpass the oxidizing ability of oxygen leads to extreme corrosivity against oxide-containing materials often thought as incombustible. Chlorine trifluoride and gases like it have been reported to ignite sand, asbestos, and other highly fire-retardant materials. In an industrial accident, a spill of 900 kg of chlorine trifluoride burned through 30 cm of concrete and 90 cm of gravel beneath.[12] Fire control/suppression is incapable of suppressing this oxidation, therefore the surrounding area is kept cool until the reaction ceases.[13] The compound reacts violently with water-based suppressors, and oxidizes in the absence of atmospheric oxygen, rendering atmosphere-displacement suppressors such as CO2 and halon completely ineffective. It ignites glass on contact.[14]
Exposure of larger amounts of chlorine trifluoride, as a liquid or as a gas, ignites tissue. The hydrolysis reaction with water is violent and exposure results in a thermal burn. The products of hydrolysis are mainly hydrofluoric acid and hydrochloric acid, usually released as steam or vapor due to the highly exothermic nature of the reaction. Hydrofluoric acid is corrosive to human tissue, is absorbed through skin, selectively attacks bone and stimulates pain nerves, and causes often-fatal fluorine poisoning. Hydrochloric acid is secondary in its danger to living organisms, but is several times more corrosive to most inorganic materials than hydrofluoric acid.
Chlorine trifluoride has been investigated as a high-performance storable oxidizer in rocket propellant systems. Handling concerns, however, prevented its use. John D. Clark summarized the difficulties:
It is, of course, extremely toxic, but that's the least of the problem. It is hypergolic with every known fuel, and so rapidly hypergolic that no ignition delay has ever been measured. It is also hypergolic with such things as cloth, wood, and test engineers, not to mention asbestos, sand, and water — with which it reacts explosively. It can be kept in some of the ordinary structural metals — steel, copper, aluminum, etc. — because of the formation of a thin film of insoluble metal fluoride which protects the bulk of the metal, just as the invisible coat of oxide on aluminum keeps it from burning up in the atmosphere. If, however, this coat is melted or scrubbed off, and has no chance to reform, the operator is confronted with the problem of coping with a metal-fluorine fire. For dealing with this situation, I have always recommended a good pair of running shoes."